Influence of the Spectrophotometer on Readings and Results Obtained from Commercial Equipment when using ELISA Post Interferon Gamma Release Assay for in vitro Diagnosis of Human Tuberculosis
Rogério Reis Conceição
DOI10.21767/2472-1948.100003
1Department of Immunology, PPGIm, ICSUFBA, Brazil
2Laboratory of Immunology and Molecular Biology (LABIMUNO) at the Health Sciences Institute (ICS) of the Federal University of Bahia (UFBA), Bahia School of Medicine and Public Health, Brazil
3OtávioMangabeira Hospital, Postgraduate Program in Organs and Systems – PIOS, Laboratory of Immunology and Molecular Biology (LABIMUNO INCT-DT Institute/CNPq/MCT, Brazil
4College of Science and Technology (FTC), Laboratory of Immunology and Molecular Biology (LABIMUNO), INCT-DT Institute/CNPq/MCT, Brazil
- Corresponding Author:
- Rogerio Reis Conceicao
Laboratório de Imunologia e Biologia Molecular. Instituto de Ciencias da Saude.
Av. Reitor Miguel Calmon s/n. Vale do Canela – 40.110-100 - Salvador-Bahia, Brazil.
E-mail: rogerioreisconceicao@gmail.com,
Fax: 55-71-3332-3341
Received date: November 06, 2015; Accepted date: February 03, 2016; Published date: February 10, 2016
Citation: Conceição RR, Freire SM, dos Santos SQ, et al. Influence of the Spectrophotometer on Readings and Results Obtained from Commercial Equipment when using ELISA Post Interferon Gamma Release Assay for in vitro Diagnosis of Human Tuberculosis. J Sci Ind Metrol. 2016, 1:3. doi: 10.4172/2472-1948.100003
Abstract
Early and reliable laboratory diagnosis is very important to guide treatment of and control strategies for a disease. Some technical problems can occur due to laboratory facilities and methodological procedures. Many in vitro diagnoses are based on colorimetric methods such as the quantitative Enzyme- Linked Immuno Sorbent Assay (ELISA), whereby optical density (OD) is compared with a standard curve. The readings of these assays are based on absorption by a spectrophotometric device of light from a narrow or broad spectrum of waves. Objective: The aim of this work was to read the ELISA plate using different spectrophotometry equipment. Methods: Human plasma samples from healthy volunteers (n=50) and volunteers with tuberculosis (n=24) were prepared previously by whole blood incubation in the commercial Interferon Gamma Release Assay (IGRA) for in vitro diagnosis of tuberculosis and then the Interferon ELISA was performed using commercial equipment from the same manufacturer. The ELISA micro plates were read in three spectrophotometers with different filters/wavelengths: 450, 630 and 450 nm. The optical densities were established and analyzed using the manufacturer’s software program. The results of the readings performed at the recommended wavelength were compared with the other two filters/wavelengths. Results: Among the results obtained, 10 samples showed differences in the results of diagnosis. These discrepancies in metrology were associated with differences in the filters/wavelengths and were consequently identified as false results.
Keywords
Spectrophotometric reading; ELISA; Spectrophotometer; Optic density (OD); Wavelength metrology
Introduction
Optical methods use the interaction of light with matter to provide qualitative and quantitative information to be analyzed. Light is characterized by photon energy, determined by frequency and wavelength. The measuring absorption or light transmission is called spectrophotometry [1]. Spectrophotometry is one of the most widely used techniques, due to the simplicity of its procedures and its speed, precision and accuracy. Moreover, spectrophotometric methods are more economical in comparison with other analytical methodologies such as chromatography and electrophoresis [1,2]. Spectrometry is objective and can be automated in various modern devices that are increasingly available and currently accessible.
The spectrometry instrument has a light source, an optical system (mono- or polychromatic), allowing the technician to choose one or more wavelengths that are focused on the sample, and a detector which generates a proportional electrical signal according to the intensity of light received [1].
The reading absorbance spectrum has been used as the basis for analytical procedures in various fields, including healthcare, where together with other techniques it assists in developing methods for diagnosing disease [3]. One test example using colorimetric and immune enzymatic techniques is the Enzyme- Linked Immunosorbent Assay (ELISA).
ELISA is widely used in medical laboratories for in vitro immunodiagnostics since it allows the detection of specific antibodies/antigens. In this technique, the polystyrene plate is used to bind antigen or antibody and a corresponding conjugate linked to an enzyme is attached. The addition of the substrate generates a colorimetric reaction that must be read by a spectrophotometer. Over the years, with variations in the methods developed different types of ELISA have been developed, such as direct, indirect, competitive and sandwich. The last two are most commonly used to detect antigens [4,5].
The sandwich ELISA is used to quantify cytokines, and in recent years it has been used for in vitro diagnosis of human tuberculosis. In this assay the cytokine Interferon gamma IFN-γ is used to identify the in vitro immune response to specific peptide antigens associated with this infection [6,7]. In the commercially available in vitro kit, whole blood of the patient is stimulated by Mycobacterium tuberculosis-specific peptides. In 24 hours, this induces the production of IFN-g in an “Interferon gamma release assay” (IGRA) test. After this first step the IFN-g is measured using an ELISA of same manufacturer. The biochemical enzymatic reaction and subsequent substrate interaction yields a colored product that is directly visualized and/or quantitatively measured by optical density [8,9]. The aim of this work was to analyze the reading of ELISA plates by using as a model of metrology analysis the readings in three different spectrophotometers and comparing results, taking in account the original spectro recommended in a newly available commercial kit used for in vitro diagnosis of human tuberculosis in Brazil.
Methods
Sample caracterization and collection
Samples were obtained from volunteers non infected [negative TST] (n=35), with active TB [positive bacilloscopy or sputum culture] (n=24) and Latent tuberculosis infection - positive TST (LTBI) (n=15) Figure 1 of one reference tuberculosis hospital (Specialized Bahia State Hospital). Samples were processed to be analyzed by Interferon gamma release assay (IGRA) in tube and Quantiferon ELISA kit (Cellestis/Qiagen-USA) for in vitro diagnosis of tuberculosis infection, as recommended by the manufacturer.
ELISA
After 24 hours incubation with each type of stimuli at the stage of the Quantiferon In tube the blood was centrifuged at 200 x g and plasma from each participant was analyzed in the ELISA plate following each step of the technical recommendations. Upon completion of the procedure, the plates were taken for reading by in three different models of apparatus and filters.
Readings were performed as recommended for the kit by using the 450 nm and 630 nm filters on the spectrophotometer I Awareness Technology (Table 1). The other two reading plates used comprised only a 450 nm filter, spectrophotometer II - Bio- Rad (iMark) (Table 2) and spectrophotometer III Thermo Scientific (Multiskan Go) (Table 3).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0,04 | 0,048 | 0,045 | 0,047 | 0,052 | 0,048 | 0,051 | 0,159 | 0,048 | 0,046 | 0,067 | 0,051 |
| B | 0,053 | 0,047 | 0,051 | 0,052 | 0,045 | 0,054 | 0,05 | 0,051 | 0,048 | 0,064 | 0,048 | 0,052 |
| C | 0,042 | 0,045 | 0,044 | 0,045 | 0,044 | 0,045 | 0,047 | 0,776 | 0,044 | 0,036 | 0,044 | 0,045 |
| D | 0,96 | 0,052 | 0,045 | 0,047 | 0,049 | 0,045 | 0,049 | 0,048 | 0,046 | 0,043 | 0,047 | 0,048 |
| E | 0,96 | 0,045 | 0,043 | 0,044 | 0,043 | 0,045 | 0,041 | 0,041 | 0,044 | 0,04 | 0,043 | 0,048 |
| F | 0,045 | 0,043 | 0,046 | 0,045 | 0,043 | 0,043 | 0,044 | 0,043 | 0,042 | 0,045 | 0,863 | 0,045 |
| G | 0,047 | 0,041 | 0,043 | 0,044 | 0,041 | 0,043 | 0,042 | 0,042 | 0,041 | 0,04 | 0,046 | 0,046 |
| H | 0,051 | 0,048 | 0,049 | 0,049 | 0,052 | 0,05 | 0,05 | 0,05 | 0,048 | 0,044 | 0,046 | 0,048 |
Table 1: Spectrophotometer I - Awareness technology (450 nm with a reference filter of 630 nm).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0,828 | 0,88 | 0,877 | 0,916 | 0,954 | 0,907 | 0,928 | 0,993 | 0,877 | 0,85 | 0,905 | 0,908 |
| B | 0,808 | 0,86 | 0,894 | 0,882 | 0,945 | 1,008 | 1,002 | 0,951 | 0,915 | 0,998 | 1,081 | 1,042 |
| C | 0,663 | 0,631 | 0,705 | 0,799 | 0,843 | 0,833 | 0,823 | 1,217 | 0,798 | 0,845 | 0,839 | 0,935 |
| D | 1,594 | 0,813 | 0,832 | 0,869 | 0,979 | 0,924 | 0,933 | 0,924 | 0,839 | 0,876 | 0,955 | 1,009 |
| E | 1,437 | 0,598 | 0,627 | 0,693 | 0,745 | 0,737 | 0,705 | 0,747 | 0,688 | 0,636 | 0,698 | 0,819 |
| F | 0,709 | 0,694 | 0,754 | 0,737 | 0,902 | 0,905 | 0,892 | 0,851 | 0,769 | 0,856 | 1,431 | 0,979 |
| G | 0,675 | 0,701 | 0,765 | 0,889 | 0,913 | 0,95 | 0,936 | 1,007 | 0,936 | 0,926 | 0,962 | 1,073 |
| H | 0,729 | 0,744 | 0,853 | 0,87 | 1,005 | 0,916 | 0,957 | 0,929 | 0,788 | 0,892 | 0,972 | 1,039 |
Table 2: Spectrophotometer II - Bio-Rad iMark (with monochrome filter 450 nm).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0,083 | 0,141 | 0,091 | 0,095 | 0,087 | 0,097 | 0,099 | 0,203 | 0,096 | 0,095 | 0,12 | 0,105 |
| B | 0,099 | 0,089 | 0,092 | 0,094 | 0,096 | 0,096 | 0,091 | 0,094 | 0,089 | 0,107 | 0,096 | 0,098 |
| C | 0,089 | 0,092 | 0,089 | 0,088 | 0,085 | 0,085 | 0,095 | 0,815 | 0,09 | 0,08 | 0,091 | 0,091 |
| D | 0,964 | 0,095 | 0,093 | 0,087 | 0,086 | 0,086 | 0,09 | 0,091 | 0,092 | 0,084 | 0,089 | 0,094 |
| E | 0,989 | 0,094 | 0,086 | 0,088 | 0,085 | 0,085 | 0,084 | 0,086 | 0,091 | 0,087 | 0,088 | 0,094 |
| F | 0,086 | 0,083 | 0,086 | 0,085 | 0,084 | 0,084 | 0,085 | 0,081 | 0,085 | 0,087 | 0,899 | 0,09 |
| G | 0,147 | 0,228 | 0,153 | 0,192 | 0,178 | 0,215 | 0,135 | 0,166 | 0,239 | 0,05 | 0,059 | 0,057 |
| H | 0,219 | 0,215 | 0,192 | 0,14 | 0,226 | 0,149 | 0,15 | 0,229 | 0,231 | 0,051 | 0,06 | 0,055 |
Table 3: Spectrophotometer III - Thermo Scientific Multiskan Go (with monochrome filter 450 nm).
After reading, the optical densities (OD) of each ELISA plate were input into the special software recommended by the manufacturer. Depending on the OD value and internal calculations of the specific program (QuantiFERON TB-Gold Analysis Software v.2.62), result sheets were generated by manufacturer, according to the internal cut off, giving positive, negative and indeterminate results.
All readings with the three different wavelengths were compared and the concordance with the readings from the analysis following manufactures instructions were calculated and expressed in percentual (%).
Results
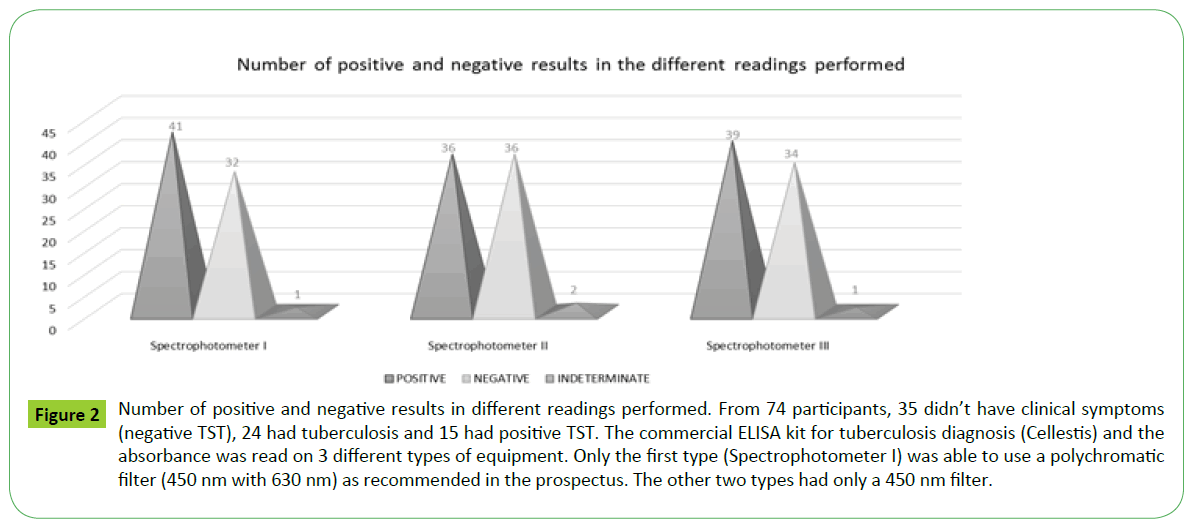
According to the results obtained from the ELISA plate reader in the spectrophotometer I Awareness Technology (450nm with a reference filter of 630 nm) (Table 4), of the total 74 samples, 41 tested positive for tuberculosis, 32 were negative and 1 indeterminate. The optical densities obtained from readings taken by the spectrophotometer II Bio-Rad iMark and spectrophotometer III Thermo Scientific Multiskan Go with monochrome filter 450nm showed different results from the first reading Figure 2. For spectrophotometer II (Table 5), 36 samples were positive, 36 were negative and 2 indeterminate. For spectrophotometer III (Table 6), 39 were positive, 34 negative and 1 indeterminate. It is noteworthy that the different readings took place within a short time. There seems to be a discrepancy in the results of three readings, and when the first is compared with the others, the number of negative results increases.
Figure 2: Number of positive and negative results in different readings performed. From 74 participants, 35 didn’t have clinical symptoms (negative TST), 24 had tuberculosis and 15 had positive TST. The commercial ELISA kit for tuberculosis diagnosis (Cellestis) and the absorbance was read on 3 different types of equipment. Only the first type (Spectrophotometer I) was able to use a polychromatic filter (450 nm with 630 nm) as recommended in the prospectus. The other two types had only a 450 nm filter.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0,003 | 0,16 | 0,134 | 0,12 | 0,131 | 0,102 | 0,119 | 0,148 | 0,206 | 0,185 | 0,005 | 0,006 |
| B | 0,007 | 0,198 | 0,16 | 0,139 | 0,169 | 0,158 | 0,129 | 0,18 | 0,158 | 0,16 | 0,006 | 0,005 |
| C | 0,006 | 0,253 | 0,127 | 0,195 | 0,137 | 0,069 | 0,105 | 0,144 | 0,263 | 0,103 | 0,005 | 0,005 |
| D | 1,904 | 0,151 | 0,123 | 1,357 | 0,152 | 0,125 | 0,083 | 0,168 | 0,233 | 0,009 | 0,005 | 0,005 |
| E | 1,371 | 0,107 | 0,13 | 0,2 | 0,2 | 0,133 | 0,121 | 0,206 | 0,264 | 0,01 | 0,005 | 0,005 |
| F | 0,106 | 0,109 | 0,175 | 0,101 | 0,101 | 0,148 | 0,153 | 0,094 | 0,276 | 0,006 | 0,007 | 0,004 |
| G | 0,105 | 0,186 | 0,111 | 0,15 | 0,15 | 0,17 | 0,093 | 0,123 | 0,194 | 0,006 | 0,005 | 0,003 |
| H | 0,174 | 0,172 | 0,139 | 0,1 | 0,1 | 0,109 | 0,111 | 0,193 | 0,191 | 0,007 | 0,004 | 0,006 |
Table 4: Spectrophotometer I - Awareness technology (450 nm with a reference filter of 630 nm).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0,7 | 0,876 | 0,824 | 0,906 | 0,98 | 0,995 | 1,043 | 1,026 | 1,082 | 1,076 | 0,151 | 0,231 |
| B | 0,763 | 0,953 | 0,798 | 0,89 | 1,058 | 0,945 | 1,099 | 0,995 | 1,042 | 1,051 | 0,145 | 0,251 |
| C | 0,599 | 0,86 | 0,688 | 0,854 | 0,882 | 0,757 | 0,873 | 0,854 | 0,974 | 0,873 | 0,128 | 0,181 |
| D | 2,072 | 0,922 | 0,764 | 1,785 | 1,024 | 0,923 | 0,948 | 0,992 | 1,059 | 0,858 | 0,15 | 0,22 |
| E | 1,758 | 0,644 | 0,598 | 0,789 | 0,85 | 0,762 | 0,772 | 0,867 | 0,939 | 0,75 | 0,154 | 0,197 |
| F | 0,776 | 0,836 | 0,826 | 0,911 | 0,962 | 0,94 | 1,01 | 0,925 | 1,096 | 0,914 | 0,167 | 0,197 |
| G | 0,802 | 0,934 | 0,721 | 0,905 | 1,045 | 0,934 | 1,011 | 0,94 | 1,057 | 0,89 | 0,173 | 0,216 |
| H | 0,983 | 0,985 | 0,808 | 0,903 | 1,159 | 0,932 | 1,118 | 1,036 | 1,024 | 0,944 | 0,167 | 0,226 |
Table 5: Spectrophotometer II - Bio-Rad iMark (with monochrome filter 450 nm).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0,083 | 0,141 | 0,091 | 0,095 | 0,097 | 0,097 | 0,099 | 0,203 | 0,095 | 0,12 | 0,12 | 0,105 |
| B | 0,099 | 0,089 | 0,092 | 0,094 | 0,088 | 0,096 | 0,091 | 0,094 | 0,107 | 0,096 | 0,096 | 0,098 |
| C | 0,089 | 0,092 | 0,089 | 0,088 | 0,085 | 0,085 | 0,095 | 0,815 | 0,08 | 0,091 | 0,091 | 0,091 |
| D | 0,964 | 0,095 | 0,093 | 0,087 | 0,089 | 0,086 | 0,09 | 0,091 | 0,034 | 0,089 | 0,089 | 0,094 |
| E | 0,989 | 0,094 | 0,086 | 0,088 | 0,086 | 0,085 | 0,084 | 0,086 | 0,087 | 0,088 | 0,088 | 0,094 |
| F | 0,086 | 0,083 | 0,086 | 0,085 | 0,084 | 0,084 | 0,085 | 0,081 | 0,087 | 0,899 | 0,899 | 0,09 |
| G | 0,093 | 0,083 | 0,086 | 0,088 | 0,086 | 0,085 | 0,09 | 0,089 | 0,086 | 0,086 | 0,095 | 0,091 |
| H | 0,095 | 0,088 | 0,089 | 0,091 | 0,095 | 0,092 | 0,091 | 0,089 | 0,088 | 0,085 | 0,09 | 0,092 |
Table 6: Spectrophotometer III - Thermo Scientific Multiskan Go (with monochrome filter 450 nm).
In the individual analysis of results, 10 participants had a different diagnosis depending on the type of filter (mono or polychromatic) and equipment used. The results obtained from the monochrome readers contradict those obtained with the recommended reader (450/630 nm) as can be seen in Figure 2.
It can be seen that when a 630 nm filter is not used, a false negative or false positive result may be obtained.
Discussion
Some technician problems can occur due to misuse of inadequate or different equipment in an indiscriminate way in the laboratory. The different spectra waves between quite similar, but not equal, spectrophotometers can generate discrepant results from the ELISA and this misuse can lead to problems with consequences that may vary between minor and major.
Fast and accurate diagnosis is the major challenge to the control of many diseases. Effective diagnosis is very important for the proper treatment of patients, whose lives may otherwise be negatively influenced [7]. Additionally, quality control of the techniques employed and the technical responsibilities of those conducting the tests are all important for reliable and true results.
Several factors can interfere with results, leading to false negatives or false positives. These can discredit the lab and influence not only the life of the patient but also that of the community in which he or she lives.
Two new studies with European population called attention of the high variability of IGRA that were not well understood and this can be a challenge to interpretation of the results. According the two research groups, this variability can occur because of the cut-off established by Manufacturer, by chance or measurement variability that can influencing the clinical post-diagnosis decisions [8,9].
These problems were identified and used to justify the differences observed in data collected in two different populations from developed countries such as Germany and France. Schablon et al and Nienhaus et al [8,9] had used in their scientific articles the same kit described in our work.
Critically, the results of IGRA tests can be influenced by different causes of the origin methodological as the stage of incubation, washing steps, measurement devices for Optical Density, calculation program, and epidemiological clinical factors (contact period and natural immunological response of the studied people).
False positives can lead to treatment of non-infected patients who will suffer adverse effects or may even go on to develop tuberculosis and with resistant mycobacteria.
A false negative individual with tuberculosis, for instance, will not participate in a treatment regimen and will then contribute to the spread of the disease [9]. Thus, a safe outcome depends on perfectly standard techniques of preparation and following the instructions of the equipment supplier.
The immunoenzymatic ELISA has been widely used in the diagnosis of various pathologies, and in this method the absorbance reading is used by the spectrophotometer to interpret results [4,5,10,11].
In this work, we used different models of spectrophotometers to compare the different absorbance readings and analyze how these different readings can influence the outcome of a diagnostic test.
From Figures 2 and 3 it can be observed that the group with negative TST showed positivity for TB according to the method used. This can be explained by the fact that these are contacts (exposure to patients with TB) and may have developed an immune response to the pathogen without developing clinical symptoms. Our data show that differences occur in the results of diagnosis due to reading plates in close waves in different spectrophotometers, even using similar equipment. Good facility and quality management and critical knowledge can lead to the minimization of mistakes and contribute toward improving quality control and the reliability of results in both the diagnostic and scientific area and to the laboratory’s success.
Ethical Aspects
The project was approved by the Comitê de Ética em Pesquisa da FundaçãoBahiana (nº119/2008) ethical committee and the volunteer participants were informed about the objectives of the trial. Peripheral blood was collected after volunteers’ signed to give their Informed Consent (IC).
References
- Rojas FSánchez, Ojeda C Bosch (2009) Recent development in derivative ultraviolet/visible absorption spectrophotometry: 2004–2008: A review. AnalyticaChimicaActa 635: 22-44.
- Morawski RZ (2006) Spectrophotometric applications of digital signal processing. Measurement Science and Technology. 17: 117.
- Mahmoud K, Park S, Park SN, Lee DH (2014) An imaging spectrophotometer for measuring the two-dimensional distribution of spectral reflectance. Metrologia 51: 293.
- Meirelles PG, Biazon L, Ono MA, Hirooka EY, Ono EY (2006) Immunoassays: an alternative for toxigenic fungi detection in foods. Semina: Ciências Agrarias
- Souza Gil E, Kubota LT, Yamamoto YI (1999) Alguns aspectos de imunoensaios aplicados à química analítica. Química Nova 22: 875.
- Teixeira HC, Abramo C, Munk ME (2007) Diagnóstico imunológico da tuberculose: problemas e estratégias para o sucesso. J Bras Pneumol 33: 323- 34.
- Campos HS (2006) Diagnóstico da tuberculose. Pulmao RJ 15: 92-99.
- Nienhaus A, Gariepy PK, Trouve C, Lhaumet C, Toureau J, et al. (2014) Tuberculosis screening at the Sainte-Anne Hospital in Paris–results of first and second IGRA. Reason 60: 4-2.
- Schablon A, Nienhaus A, Ringshausen FC, Preisser AM, Peters C (2014) Occupational Screening for Tuberculosis and the Use of a Borderline Zone for Interpretation of the IGRA in German Healthcare Workers.
- Jamal LF, Moherdaui F (2007) Tuberculose e infecção pelo HIV no Brasil: magnitude do problema e estratégias para o controle. Revista de Saúde Pública 41: 104.
- Peruski AH, Peruski LF (2003) Immunological Methods for Detection and Identification of Infectious Disease and Biological Warfare Agents. Clinical And Diagnostic Laboratory Immunology 10: 506–513.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences